ID.Flow® - a carefully designed test system using whole blood.
Understanding human immune responses during non-clinical development is challenging.
It requires careful analysis of the drug’s impact on various cells and blood components, as well as potential downstream interactions. We are proud to present our test system, ID.Flow, which can detect immune responses that are difficult to assess in plate-based assays and animal models.
Trusted by drug developers across the globe.






Features

DONORS
We draw blood from donors directly at our test site allowing us to observe the effects of drugs on fresh, minimally manipulated whole blood, offering our customers invaluable insights into how their drugs affect complex biological systems in the blood.

RELEVANCE
ID.Flow is a highly advanced test system that ensures the continuous circulation of blood and test substance at a regulated temperature of 37°C. Its physiological features set it apart from other assays such as traditional PBMC and whole blood assays.

COMPLIANCE
Regulatory authorities encourage the use of New Approach Methodologies (NAMs) to evaluate the immunotoxicity potential of new drugs. ID.Flow is a NAM that can help in reducing and refining animal studies.
ID.Flow®
Explore the readouts
Cytokine Release
Sensitive cytokine release prediction in fresh human whole blood.
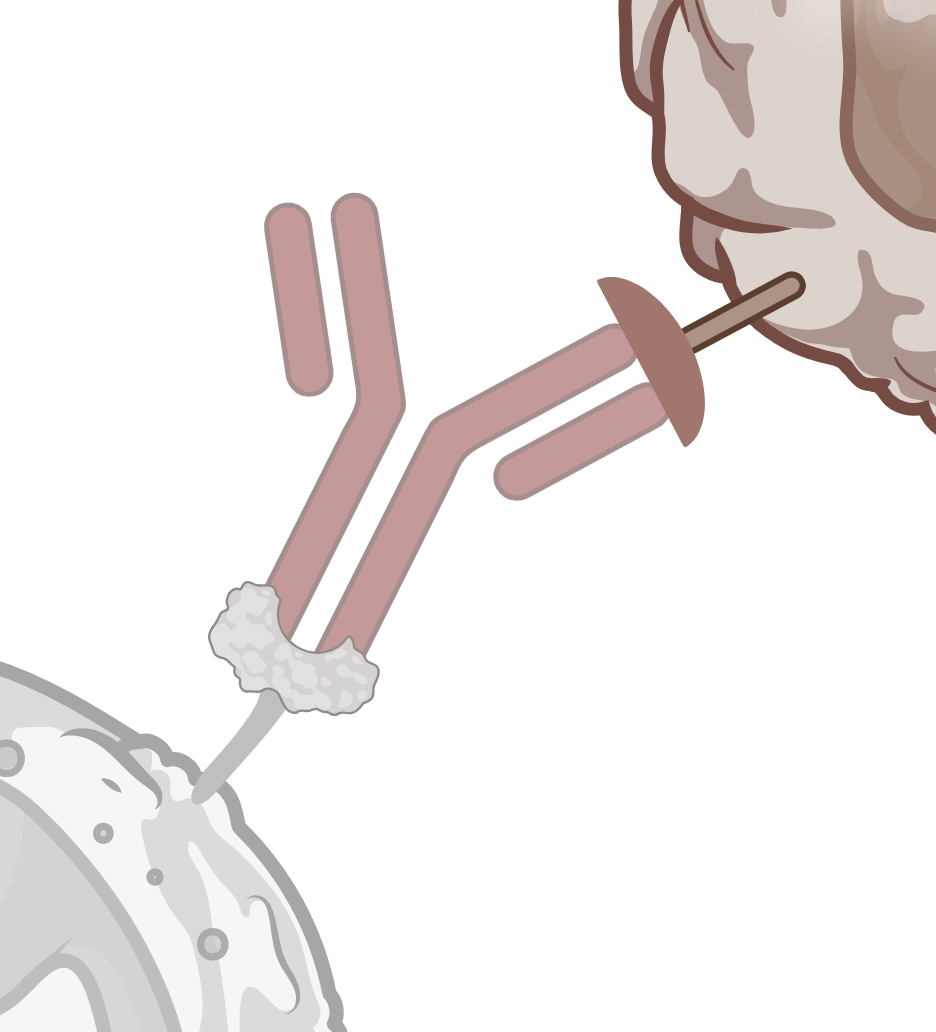
ADCC / CDC
Simultaneous study of ADCC and CDC in fresh human whole blood.
Complement Assays
Assessment of C3a, C5a, and Bb split product in fresh human whole blood with active complement.
White Blood Cells, Activation & Depletion
Activation of B cells, T cells, NK cells, monocytes, and granulocytes is simultaneously analyzed in fresh human whole blood.
Platelets, Count & Activation
Platelet activation, conjugates, aggregation, counts, inhibition...
Frequently asked Questions
No items available