Therapeutic Oligonucleotides
Leverage our human-relevant assays in preclinical oligonucleotide development.
Comprehensive Safety Assessment in Human Whole Blood
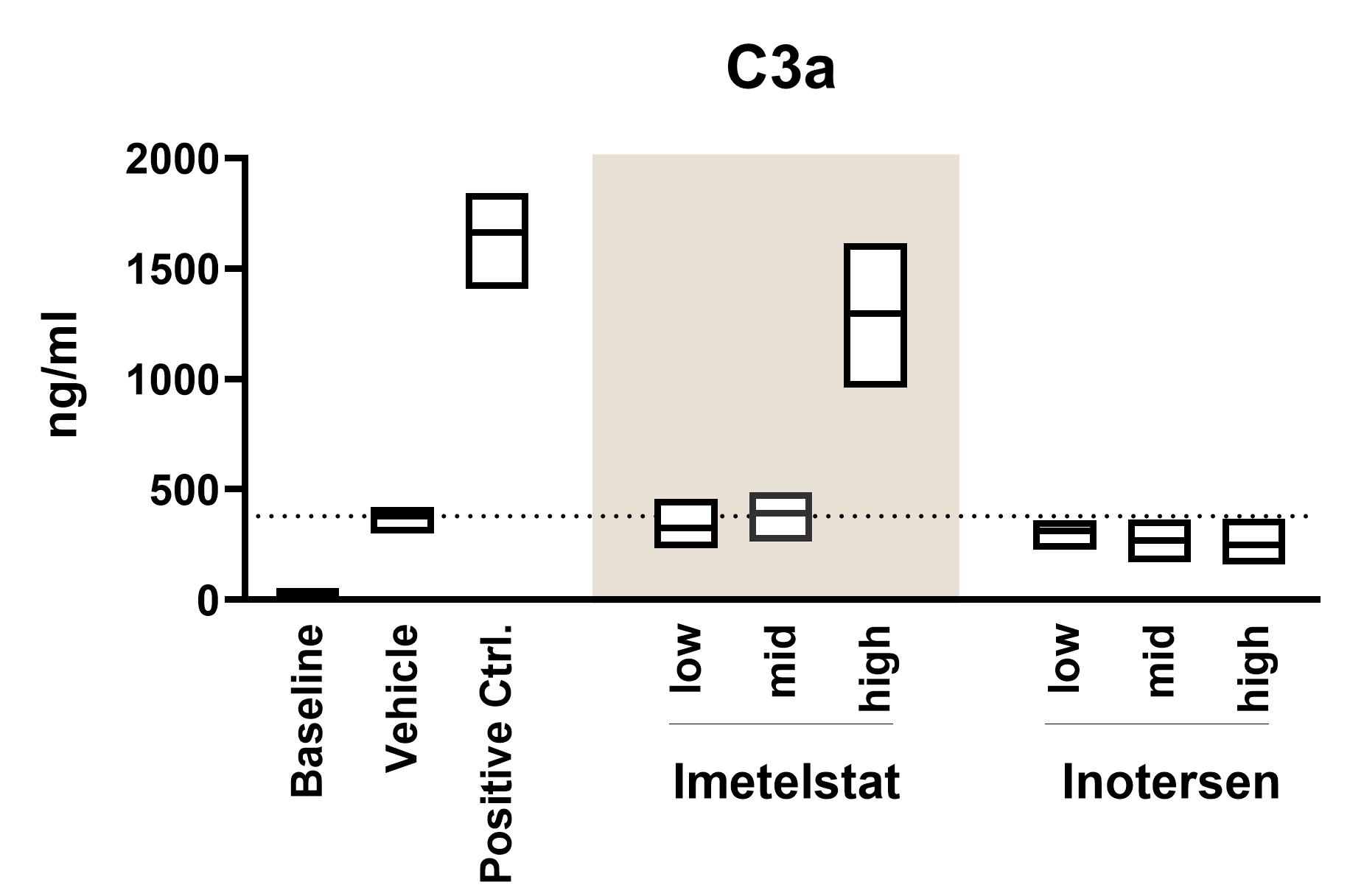
Complement Activation
Complement activation can have deleterious consequences. Our assays measure the activated complement fragments C3a, C5a and the Bb split product, in accordance with FDA guidelines.
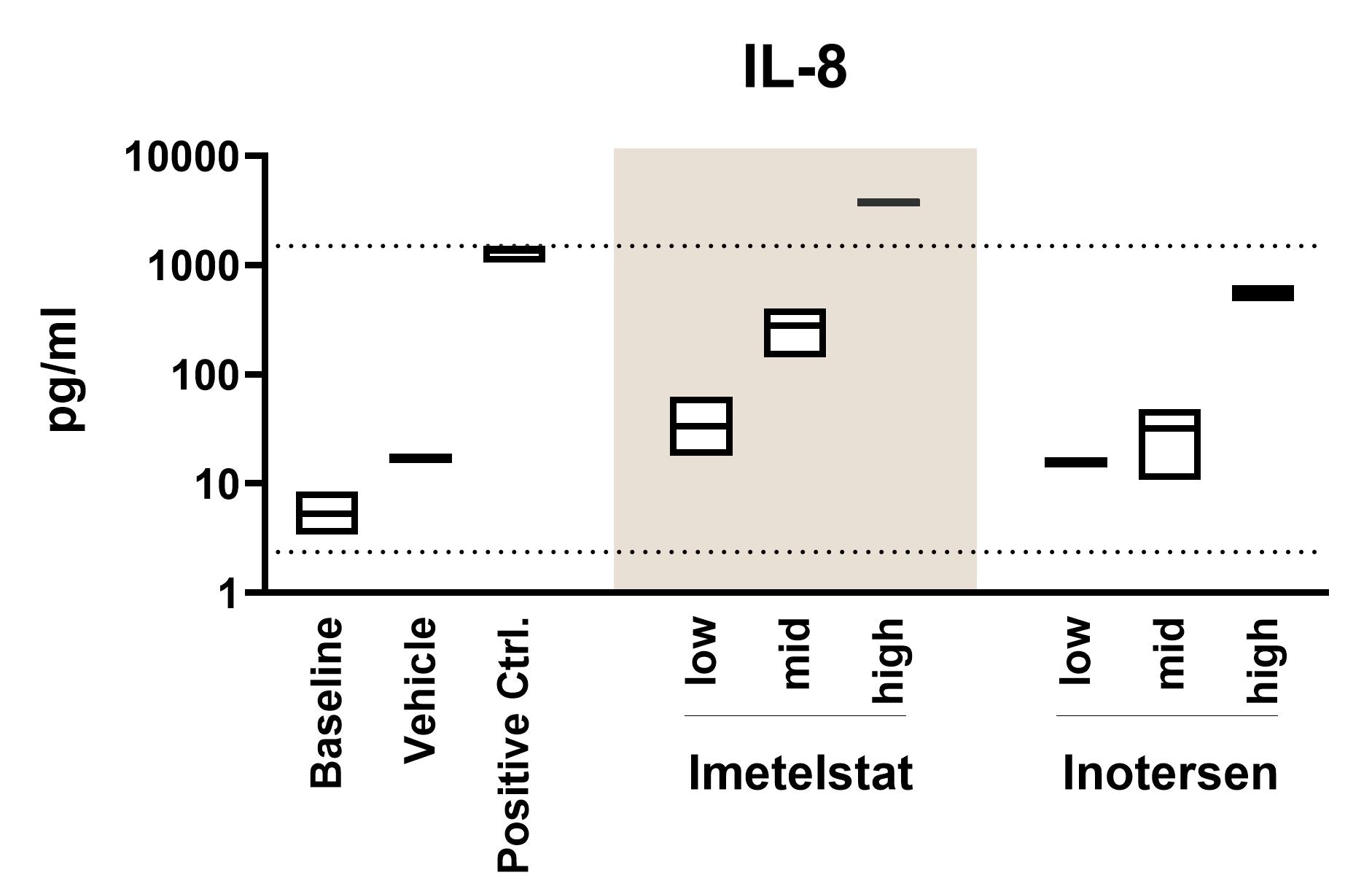
Cytokine Release
Activation of the innate and adaptive immune responses results in cytokine release, detected using a sensitive multiplex assay.
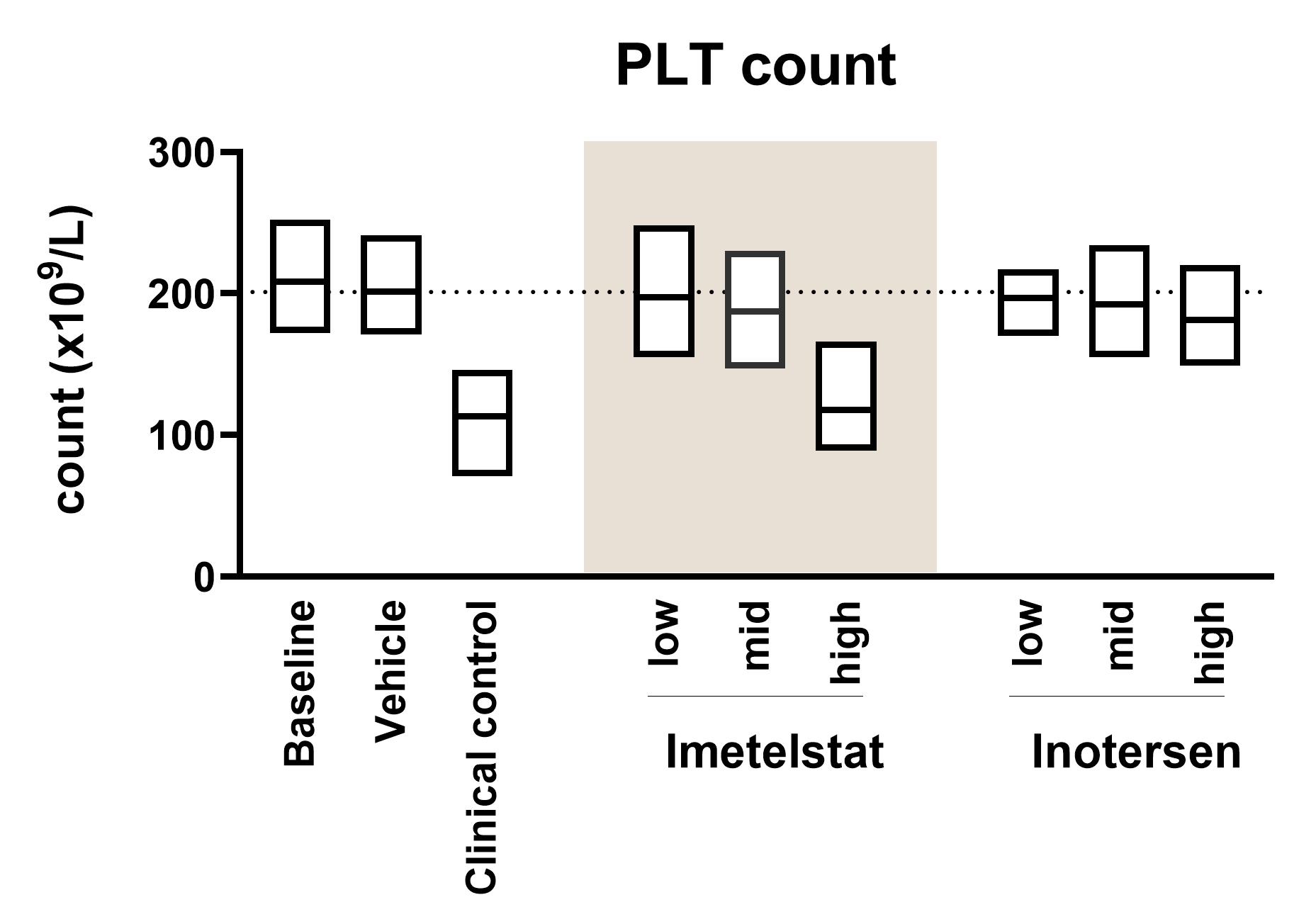
Platelet Activation
Flow cytometry is used to detect platelet aggregation, activation marker expression, and the formation of platelet-leukocyte conjugates.
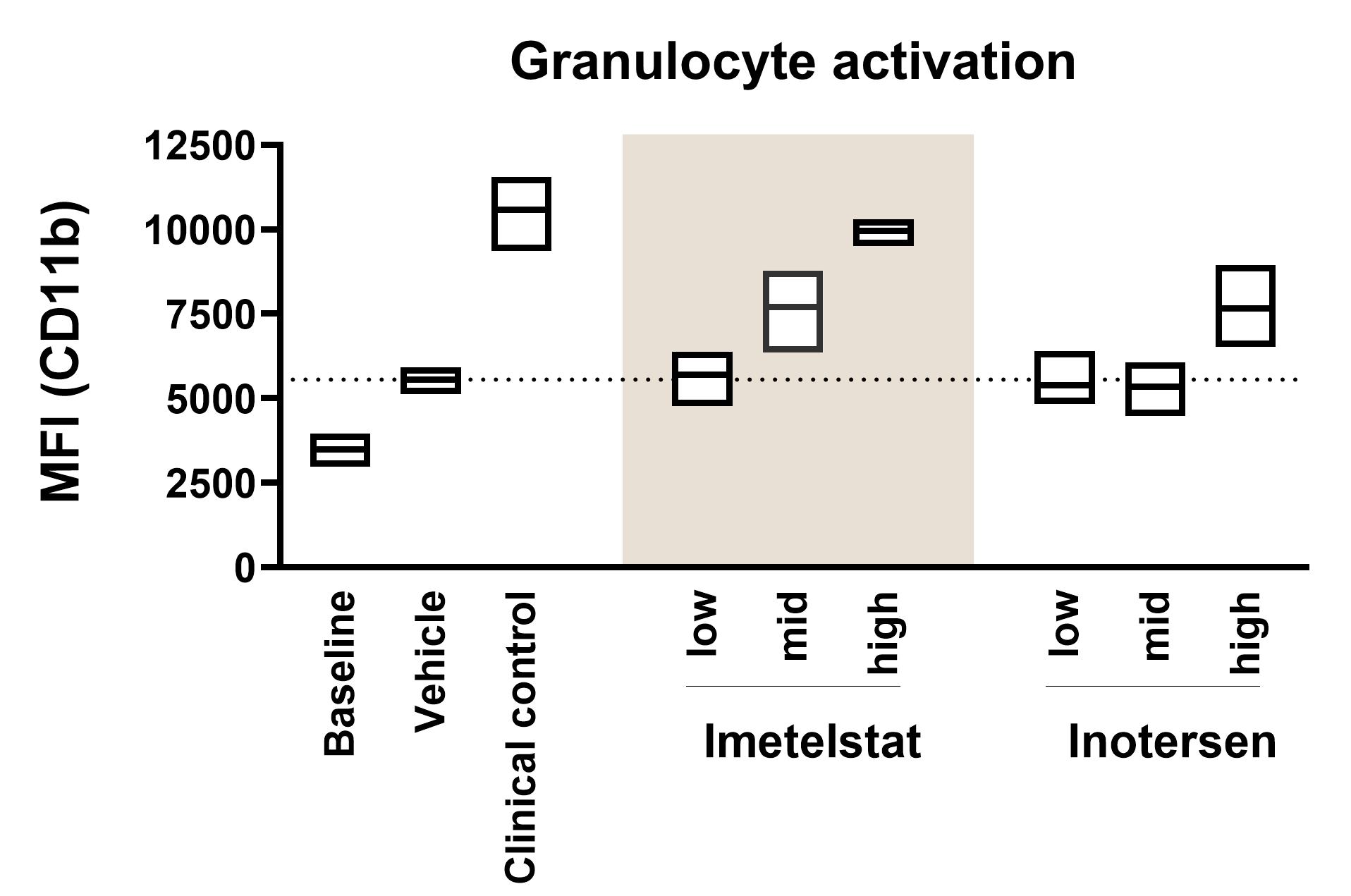
Cell Activation & Depletion
The numbers and activation status of major white blood cell populations: granulocytes, monocytes, T cells, B cells, and NK cells, are measured using flow cytometry.
Haemolysis & Clotting
The grade of haemolysis and clotting is determined by visual inspection.
Blood & Immune Cell Count
Red blood cells, white blood cells, and platelet counts are measured using an automated differential haematology analyser, to assess gross effects on the major cell types in blood.
ASSAYS
Accurately predict human immune responses for oligonucleotide-based drugs.
The development of oligonucleotide-based drugs (ONDs) presents unique challenges in preclinical immunotoxicology testing. Traditional models, such as non-human primates, often fail to capture critical human immune reactions, leading to incomplete safety assessments and unforeseen risks in later development stages. Immuneed’s test system ID.Flow® offers a human-relevant environment to assess key immunotoxicology markers, enabling physiologically relevant assessments and benchmarking against relevant clinical controls.
Testing for immunotoxicity requires a human-relevant model.
Assays based on fresh human whole blood are particularly suitable for predicting how the human immune system will respond to an OND in terms of immunotoxicity. Immuneed offers comprehensive services to assess the safety of oligonucleotides. All our services are based on our innovative test system ID.Flow. The ID.Flow is capable of assessing over 40 readouts from every individual sample and it produces a wide range of data for each test item, below are a few examples:
Recommended Readouts
- Complement activation: To assess complement activation, fresh human whole blood is incubated with test substances in ID.Flow and then analysed by measuring the complement split products C3a, C5a, and Bb using ELISA assays. See the graph above for an example of C3a analysis.
- Cytokine release: Cytokine release syndrome can cause severe inflammation and organ damage. To assess cytokine release potential, fresh human whole blood is incubated with test substances in ID.Flow. Subsequently, samples are analysed using the multiplex Mesoscale Discovery (MSD) platform. Multiple time points and any cytokine available for MSD-analysis can be included. See the graph above for an example of IL-8 analysis.
- Platelet activation: We offer advanced flow cytometry-based assays to evaluate the risk of effects on platelets, such as thrombocytopenia, induced by ONDs. The assessment involves incubating fresh human whole blood with test substances using the ID.Flow® test system. Samples are analysed by flow cytometry to detect critical platelet-related adverse effects, including platelet aggregation, activation marker expression, and the formation of platelet-leukocyte conjugates. Platelet counts are also measured using a Sysmex haematology analyser, which provides quantitative data on potential reductions in platelet numbers (see the graph “PLT count” above).
- Cell activation & depletion: To assess whether an OND induces activation or depletion of various cell types, fresh human whole blood is incubated with test substances in ID.Flow. Our custom flow cytometry panel is designed to detect changes in cell activation and quantify any reduction in cell populations of B cells, T cells, NK cells, monocytes, and granulocytes. Samples can also be analysed at multiple time points to track changes over time. See above for an example of granulocyte activation analysed by flow cytometry.
- Haemolysis & Clotting: The evaluation of haemolysis and clotting is critical during preclinical safety assessment of ONDs. This is particularly important for generic ONDs where the presence of excipients in the formulation can significantly impact these parameters. The grade of haemolysis and clotting is determined by visual inspection
- Blood cell count: A reduction in cell count can indicate adverse reactions in humans, such as neutropenia or thrombocytopenia. Samples generated using ID.Flow are analysed using a Sysmex Hematology Analyzer. Besides routine measurements of white blood cells, red blood cells, and platelets, differential cell counts can also be generated to assess the effects on neutrophils and other cell types.