We design and deliver full-range platelet assessments.

COUNT
The platelet count measures the number of platelets in an individual's blood.
CONJUGATES
The formation of platelet-leukocyte conjugates reflects platelet activation.
ACTIVATION
Activated platelets produce soluble factors and directly interact with immune cells, thereby promoting inflammation.
AGGREGATION
Measures the ability of platelets to adhere to each other and form a clot, which is the key component of primary hemostasis.
INHIBITION
Platelet inhibition interferes with activation and aggregation components and reduces the incidence of thrombosis.
CLOTTING
Occular measurement of clotting.
ASSAYS
Why choose platelet assays based on whole blood?
- Human whole blood preserves complex interactions between platelets, immune cells, and other blood components.
- Provides insights beyond animal models and in vitro methods.
- Delivers clinically relevant data for preclinical safety evaluation.
- Identifies platelet-related risks, including thrombocytopenia, thrombosis, and immune-mediated effects.
Platelets – a key part of the non-clinical safety assessment.
Thrombocytopenia is among the most frequently observed drug-induced hematologic toxicities in clinical settings. To mitigate this risk, evaluating a drug’s effect on platelets is essential during the preclinical safety assessment. Early detection of potential platelet-related toxicities ensures safer and more effective drug development, reducing the likelihood of adverse effects in clinical trials.
Drugs that activate platelets can trigger a cascade of biochemical and cellular events. Platelets are metabolically active cellular fragments rich in surface receptors and granules. Upon activation, platelets undergo morphological changes, adopting an irregular shape and increasing their surface area, which enhances their aggregation potential. Platelet activation also induces the release of granule contents, including ADP and other platelet-activating molecules. This creates a feedback loop that further amplifies platelet activation. This chain reaction can rapidly lead to thrombosis, but this is just one of several platelet-related risks that can arise during drug development.
Assessment in human whole blood.
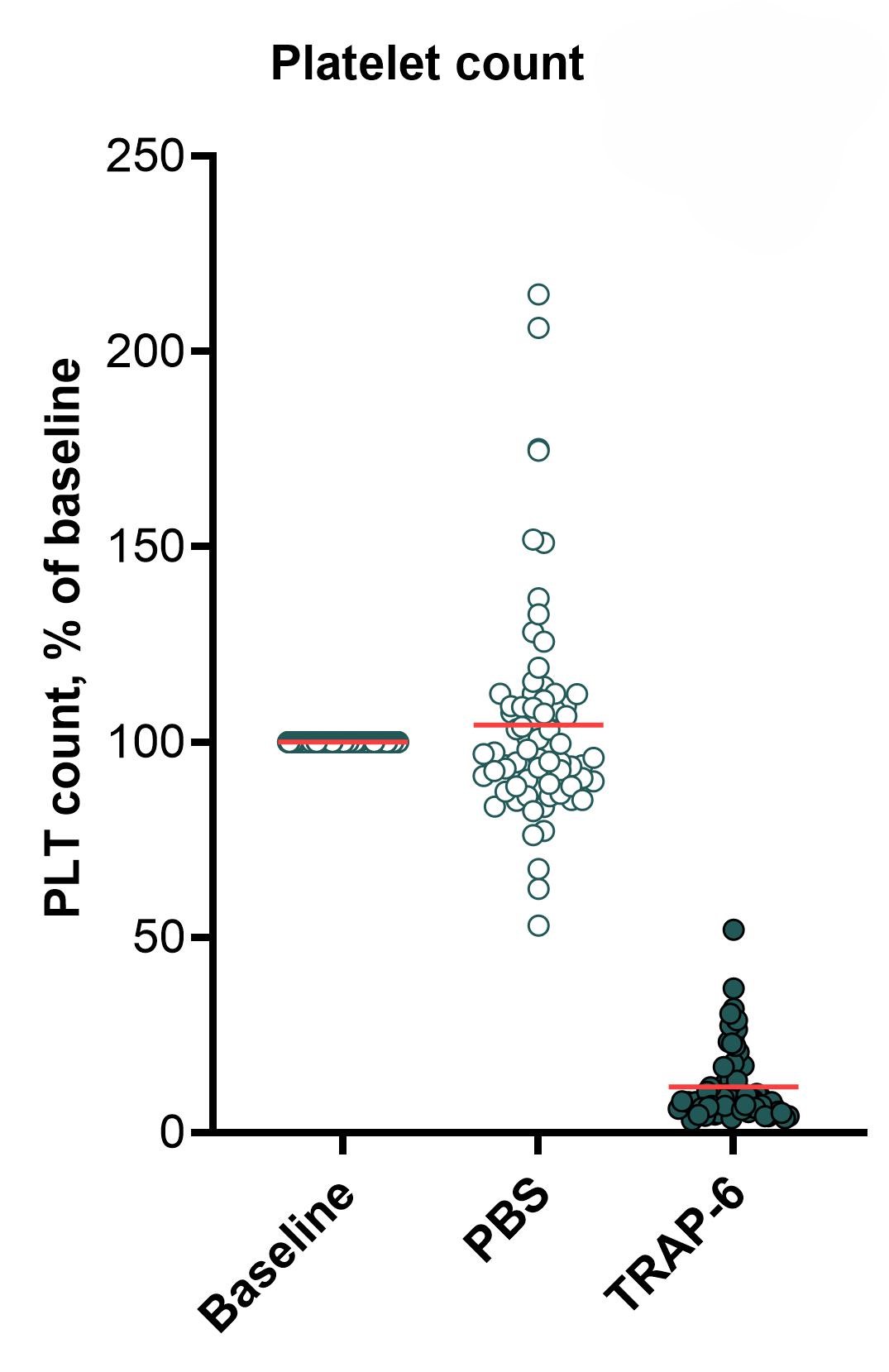
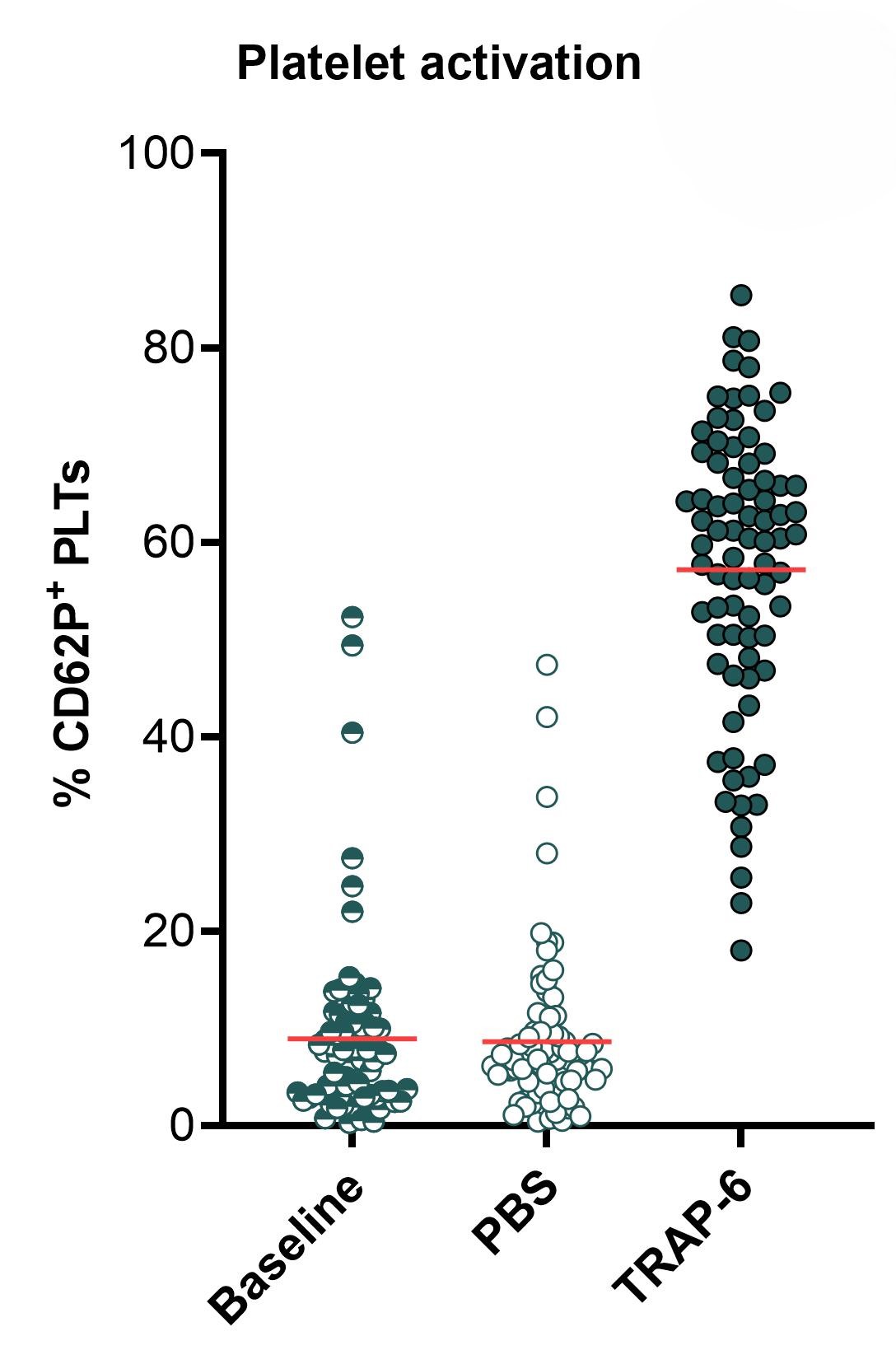
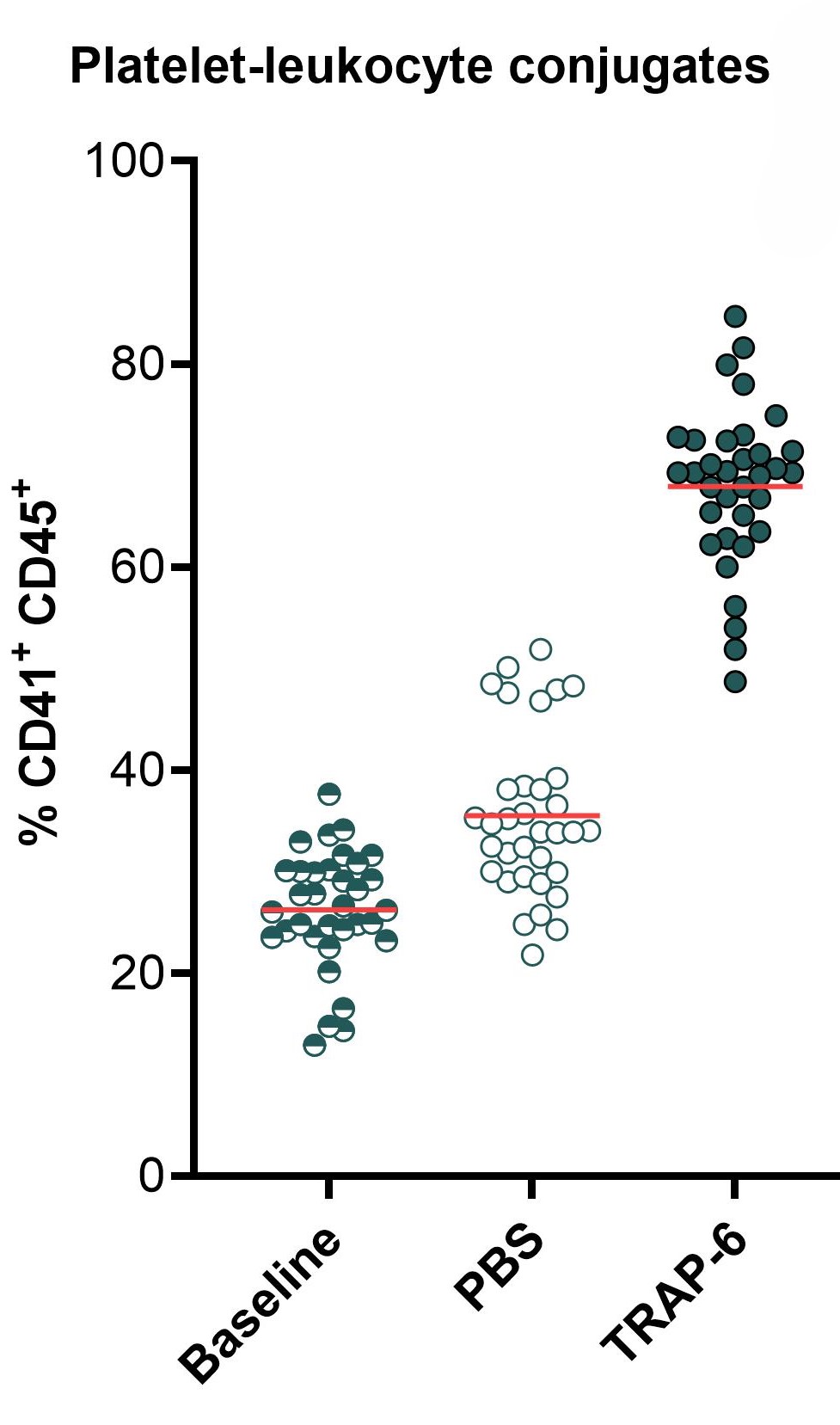
The graphs below show a flow cytometry-based assessment of platelet activation, as measured by (from left to right) platelet (PLT) count at 15 min, expression of CD62P at 1 hour, and frequency of white blood cell-platelet (WBC-PLT) conjugates at 1 hour. Platelets present in whole blood are directly activated by thrombin receptor-activating peptide-6 (TRAP-6), resulting in platelet aggregation (seen as a decrease in platelet count), expression of activation markers such as CD62P, and formation of WBC-PLT conjugates. The mean for each group is shown as a red line. Statistical analysis was performed using paired Student’s t-test, **** p < 0.00001.
More Assays
Cytokine Release
Sensitive cytokine release prediction in fresh human whole blood.
White Blood Cells, Activation & Depletion
Activation of B cells, T cells, NK cells, monocytes, and granulocytes is simultaneously analyzed in fresh human whole blood.
ADCC / CDC
Simultaneous study of ADCC and CDC in fresh human whole blood.
Complement Assays
Assessment of C3a, C5a, and Bb split product in fresh human whole blood with active complement.