Safety and efficacy in immune-oncology – getting the balance right.
July 2, 2021
Attempting to harness the body’s adaptive defense system into a drug?
In this post, discover different factors to consider when trying to strike the right balance between the safety and efficacy of your antibody.
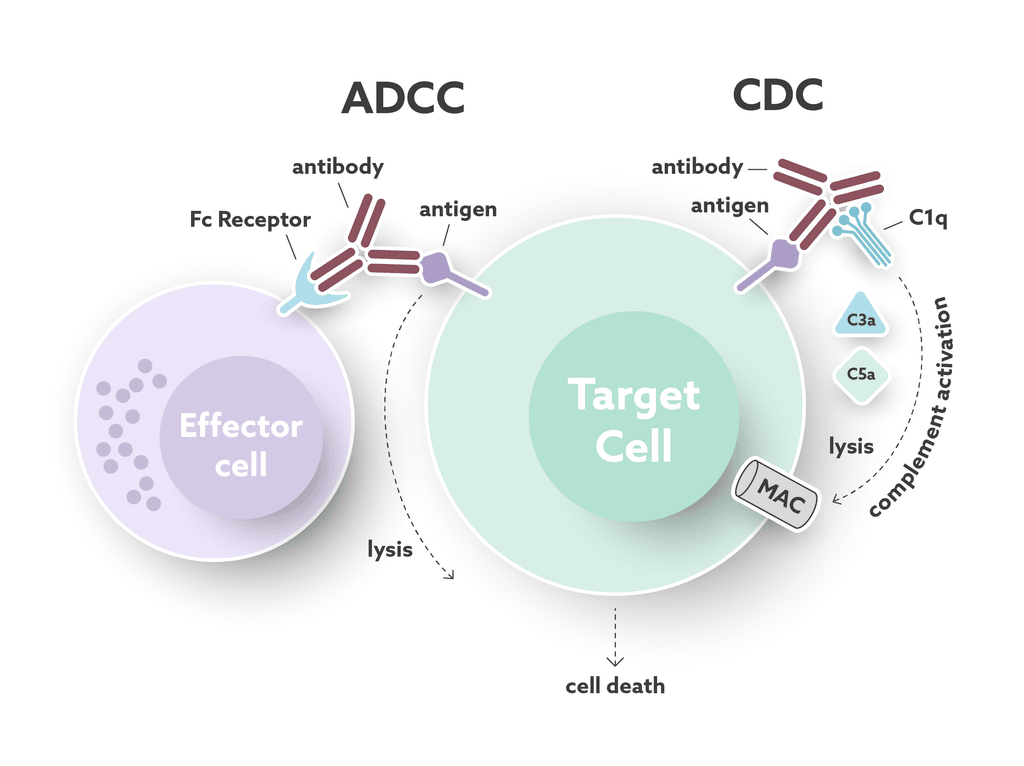
Antibody effector functions play an important part in the humoral immune response. These functions form an essential link between the innate and adaptive immune systems. Most of the effector functions are induced by the antibody’s “tail”, the fragment crystallizable (Fc) region. The Fc region can interact with complement proteins and specialized Fc-receptors to mediate cell cytotoxicity (see Fig.1). Immuno-oncology is a field of research that utilizes the natural properties of antibodies to develop targeted cancer treatments. While these immunomodulating drugs can kill, or stimulate the killing of cancer cells, they also carry an inherent risk of triggering unwanted immune-mediated reactions. For this reason, it is important to fully understand the mechanisms behind both the wanted and the unwanted effects on the immune system, to strike the right balance between safety and efficacy for antibody therapeutics.
Key areas of interest when evaluating the efficacy and safety of antibody therapeutics
When an antibody therapeutic binds to its target antigen/s, the risk of inducing toxicity is dependent on many different factors – not only on the pharmacological effects induced by binding. The potential for unwanted effects depends on if the target is expressed in healthy organs and tissues and to which extent the binding to the target antigen interferes with normal physiological processes. An antibody targeting an antigen that is expressed on both cancer and normal cells and that is involved in normal cell function, such as rituximab (anti-CD20), will have higher potential toxicity than an antibody against an antigen that has a restricted tissue expression or function. If the target receptor is well-defined in terms of its biology and tissue distribution, one can often identify and predict potential organs of toxicity. For antibodies, the choice of IgG isotype and the design of the Fc region will also have a major impact on efficacy and safety, since the latter both mediates important antibody effector functions and influences the circulating half-life of the antibody.
In the last decade, a wide range of bioengineering strategies has been developed with the aim of improving the risk-benefit profile of antibodies as cancer treatments (see next section). Strategies for improving efficacy include the use of antibody-drug conjugates (ADC), antibodies directed towards multiple targets simultaneously (bi-specific antibodies) or having several binding sites (multivalent antibodies). Measures to improve safety include steering the plasma half-life of the antibody or tailoring the cytotoxic response by making changes to the Fc-binding part.
Despite these advances, the need to thoroughly understand and optimize the risk-benefit ratio of engineered antibodies remains. Changing the Fc region of an antibody to enhance complement-dependent cytotoxicity (CDC) may make it more immunogenic as a result of its physicochemical properties or sequence. The same applies to antibody-drug conjugates. Multiple binding sites may make the antibody more effective, but it may also make it more prone to form immune complexes. An antibody designed to enhance Fc-mediated antibody-dependent cellular cytotoxicity (ADCC) may stimulate excessive cytokine release from several effector cells or lead to a changed degradation pattern.
Bioengineering strategies for improving the safety and efficacy of antibody therapeutics
Antibody-drug conjugate (ADC): An antibody that targets cancer cells and has been linked to a cytotoxic molecule, such as a radioactive ligand or a cytostatic small molecule.
Multivalent: An antibody that can bind several target antigens on the cell surface at the same time, and enhance antibody complex formation on the cell surface to attract immune effector cells.
Bi-specific: An antibody that binds a target on a cancer cell and an immune effector cell at the same time, to bring the two cells in close proximity to each other.
Fc-engineered/Fab fragments: An antibody with point mutations in the Fc region or a deleted Fc-region that will either prevent or enhance Fc-receptor binding.
Key areas to evaluate when optimizing an antibody
- Target antigen expression and distribution in health and disease
- Fc-dependent effects such as Fc-mediated cellular activation, cytokine release, complement activation, and antibody clearance
- Product-related effects including sequence, placement of binding sites, and physicochemical properties affecting immunogenicity and clearance
Once all of these properties have been optimized, choosing the right dose for human trials may still be a challenge, but you will have a good understanding of which effects to expect. You are welcome to contact us at Immuneed to find out more about how we help companies to assess the safety and efficacy of different antibodies. Contact us here.

Elisabet is a Scientific Business Manager at Immuneed, with a strong background in Pharmaceutics and team leadership. Previously, she worked as Business Advisor for Uppsala University Innovation and brought her strong knowledge in clinical drug development and medical affairs with her to Immuneed. She graduated from Uppsala University with a Ph.D. degree in Pharmaceutics.